Our Research
Our Cardiovascular Engineering Laboratory conducts research in the field of cardiovascular engineering, biology and physiology, imaging and medical device development. Cardiovascular disease is the leading cause of death in the industrialized world. Sudden cardiac death occurs due to the development of fatal disturbances of cardiac rhythm known as arrhythmias. The research in the Efimov laboratory is focused on advancing our understanding of the fundamental mechanisms of arrhythmogenesis, and on developing novel diagnostic tools and lifesaving anti-arrhythmic therapies. We work on development of novel biological and devices therapies, including low energy electrotherapy of arrhythmia, stretchable and flexible electronics platform, and cell reprogramming strategies. Our basic and applied research is particularly focused on investigation of the human heart physiology and pathophysiology, which provides a clinically relevant platform for novel theory development and high throughput drug screening for cardiac toxicity.
PROJECTS
BIOELECTRONICS
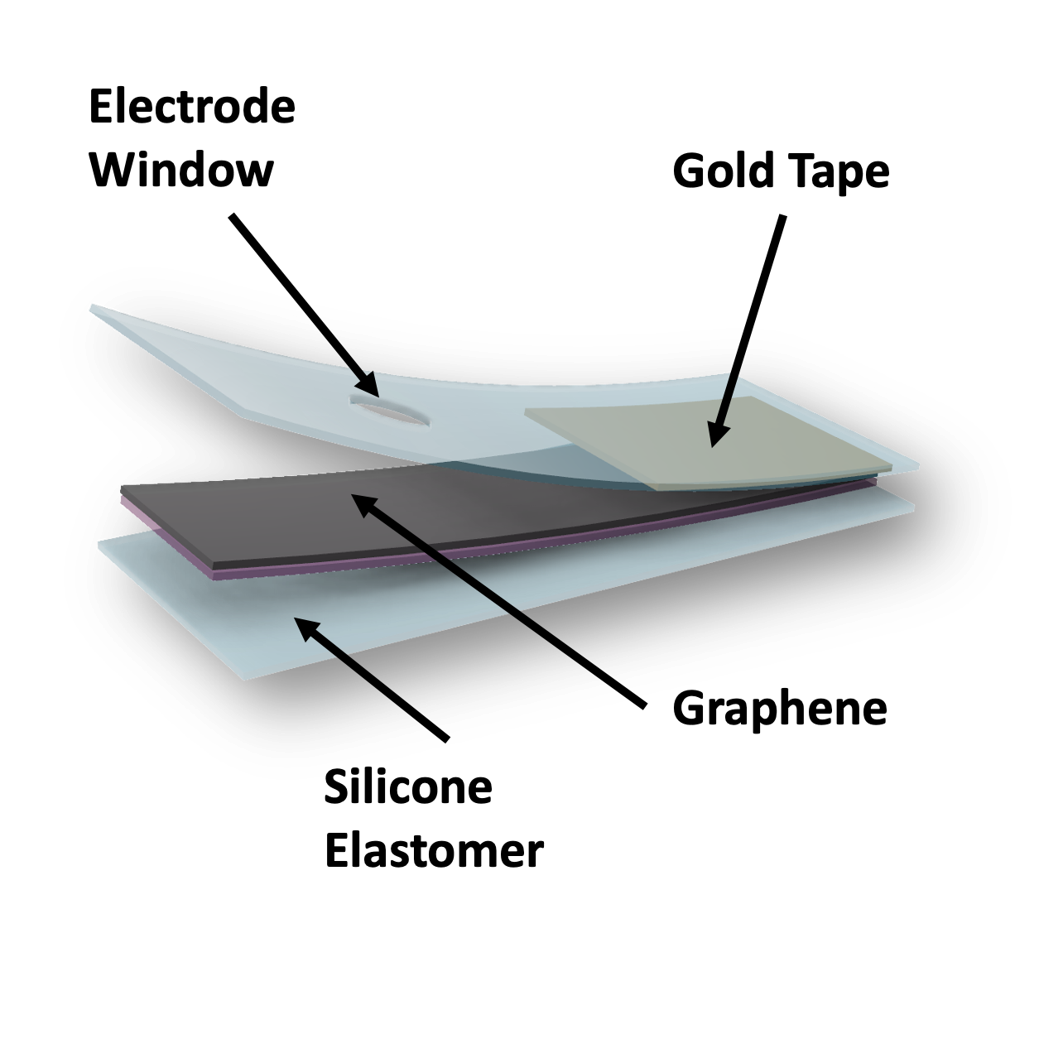
Graphene-Based Biointerfaces
The soft and lightweight biointerfaces are made of graphene using a simple and scalable methods. These devices enable high-fidelity recording of cardiac electric activities (i.e., sinus rhythm, irregular heart beats) as well as effective cardiac pacing when being placed onto a heart. The transparency of the graphene also allows the incorporation of such a device with optocardiography (i.e., using light to track and modulate heart rhythm) that further expands application scenarios.
Multifunctional Flexible Electro-Optical Colocalized Arrays
Soft, scalable, and multifunctional cardiac technology integrating multilayered colocalized m-electrodes and m-LED arrays for site specific optogenetics and electrical modulation. Ex vivo and in vivo demonstrations support functionalities in cardiac mapping and stimulation, with purposes in basic to clinical cardiology.
Wireless, Fully Implantable Pacemakers
Observation and regulation of cardiac function are critical to understanding cardiac mechanisms in both physiology and pathophysiology. Demonstrating control is essential to developing effective novel therapies. Here, we show multiple devices developed that are fully implantable and wireless. The Efimov lab has worked in multiple collaborations to develop next generation state-of-the-art technology for therapeutics and discovery.
A channel is in stimulating mode
An optical photo of the device onto a rat heart
Molybdenum and Poly(Lactic-Co-Glycolic Acid) (PLGA) Based Biointerfaces
A high-density multi-channels device is made of PLGA and molybdenum. Such a device can provide a multiplex mapping capability of cardiac electric activities and by switching different channels into stimulating mode, it can pace the heart from different cardiac regions.
The device is made biocompatible and bioresorbable such that it won't hinder the contracting heart inside the body. By metabolism, the implanted device will be dissolved inside the body which eliminates the surgery procedure of taking it out when device implantation is just a temporary requirement.
Development of a pacing-ablation-mapping (PAM) catheter for resynchronization therapy
Cardiac resynchronization therapy (CRT) is a widely used effective treatment modality for patients with symptomatic drug-refractory heart failure, but 30-40% of those treated do not respond favorably. Conduction-system pacing is a more physiological approach to CRT because of its ability to overcome limitations of biventricular pacing by directly engaging the intrinsic electromechanical sequence of the heart.
A novel conformal bioelectronic device is being developed to precisely map, ablate, and achieve selective left bundle branch pacing in which electrical activation will occur directly over the His-Purkinje system utilizing the integrated balloon pacing-ablation-mapping (PAM) catheter delivery system
(A) Optical image of the instrumented catheter inserted into a transparent heart model. (B) Schematic illustration of the stacked device arrays, complete with electrodes, temperature sensors, and pressure sensor. (C) Optical images of the electrode arrays transferred onto a silicone balloon catheter. (D) Optical images of the temperature sensors transferred onto a polyurethane balloon catheter.
ARRYTHMIA MECHANISMS
Ultra-low energy electrotherapy for treatment of cardiac arrhythmias Overview
RHYTHM 2.0 is a panoramic imaging toolkit that offers alternative methods for conducting experiments and custom-build MATLAB software for data processing and analysis. Users can generate 3D visualizations of different sized hearts ranging from mouse to rabbit and project optical data onto the meshes using user-friendly graphical user interfaces. This is useful in viewing how signals travel throughout the heart, especially in arrhythmia studies.
Specifications
Currently functional on Mac OS (not tested on Windows). Must have MATLAB installed prior to using the software.
Download
RHYTHM_v2.zip
Download link coming soon.
Installation
Visualization Toolkit Installation Manual (Mac OS) coming soon.
Visualization Toolkit Installation Manual (PC)
Manual
RHYTHM 2.0 User Manual
Attachments:
Suppressing Calcium Leaks by Targeting Ryanodine Receptor 2 in the Heart reduces Arrhythmia Burden
Conditions that lead to structural heart disease such as myocardial infarction is associated with leaky Ryanodine receptor 2 (RyR2) which increases arrhythmia susceptibility. In this study, we hypothesized that inhibiting RyR2 receptors in these hearts can reduce the arrhythmia burden. In this collaborative project with the Vanderbilt University, RyR2 inhibition was accomplished by using Dantrolene, a pan RyR2 inhibitor, and the mechanisms underlying reduced arrhythmia propensity were determined in mouse hearts and in human cardiac slices.
Mouse models of myocardial infarction and human cardiac slices treated with caffeine (to simulate leaky RyR2) were used in this study. During -adrenergic stimulation, action potential duration was reduced and arrhythmia incidence was increased in mouse hearts (ventricular tachycardia) and human slices (premature ventricular contractions, PVCs). Dantrolene treatment reduced arrhythmia incidence in these models and also restored action potential duration. Therefore, RyR2 inhibition with Dantrolene reduced arrhythmia burden by suppressing arrhythmogenic triggers and reversing arrhythmogenic substrate.
CARDIO-ONCOLOGY
p38 Mitogen-Activated Protein Kinases (MAPKs) Modulate doxorubicin cardiotoxicity in an isoform-and sex-specific manner
Doxorubicin is an anthracycline antibiotic used in cancer chemotherapy. Despite its well-documented cardiotoxic effects, it is still widely used in the clinic by limiting the therapeutic dosage. It is therefore crucial to identify strategies to protect the heart from the life-threatening side effects of doxorubicin. To identify cardioprotective approaches, it is first necessary to understand the mechanisms that underlie cardiotoxicity. Doxorubicin-induced cardiotoxicity (DIC) is associated with increased production of reactive oxygen species (ROS), activation of stress signaling pathways, DNA damage, metabolic dysfunction, and mitochondrial damage. Stress-activated signaling molecules, particularly p38 MAPKs, are well-known regulators of DIC. However, the isoform-specific roles of the four mammalian p38 MAPK family members, p38α, p38β, p38γ, and p38δ, in DIC remain incompletely understood. Our research aims to elucidate the roles of p38 MAPK isoforms in DIC, using both generic and pharmacological approaches.
We have found that systemic genetic deletion of p38δ protected female mice against DIC, improving survival and cardiac function, reducing fibrosis, and increasing autophagy. In contrast, deletion of p38γ had no significant effect on DIC. Additionally, we found that cardiomyocyte-specific deletion of p38α is protective in male mice, whereas systemic deletion of p38β was detrimental in female mice during DIC. We are currently investigating the therapeutic potential of RNA interference (RNAi)-mediated p38δ silencing for mitigating DIC in female mice and are working to translate these findings to human physiology using an ex vivo human left ventricular cardiac slice model.
CARDIAC GENOMICS
Gene expression, which governs protein expression and other cell processes, is aberrant in heart failure. The network of regulatory elements comprising promoters and enhancers controls gene expression. Both promoters and enhancers are cis-regulatory elements located near their target genes. Promoters encompass gene transcription start sites and enhancers are additional regulatory elements for further enhancement and tuning of promoter activity in a cell type-specific manner. A significant fraction of enhancers are transcribed and may share promoter features. Deeper knowledge of the dynamic transcriptional activity of promoters and enhancers is needed to improve the mechanistic understanding of the pathogenesis of heart failure, atrial fibrillation, and myocardial infarction. Our open-access heart CAGE atlas serves the cardiovascular community in improving the understanding of the role of the cardiac gene regulatory network in cardiovascular disease and therapy.
Zenbu open-access platform: https://fantom.gsc.riken.jp/zenbu/reports/#Atlas%20of%20 cardiac%20 promoters%20and%20 enhancers
HUMAN HEART CARDIAC PHYSIOLOGY
Sex Differences in Human Hearts
Increasing amounts of evidence are coming out to support sex differences in disease and its symptoms, prevalence, age of onset, and response to therapies. Such sex differences have been related to sex chromosomes, sex hormones, or other gender-related behaviors, yet the molecular and functional mechanisms underlying these differences mostly remain unexplored. Heart failure and other cardiomyopathies have distinct presentations in males versus females that are often overlooked, leading to ineffective treatment and contributing to the growing mortality from heart diseases. Understanding the differences in the pathogenesis of heart disease in males and females can guide early diagnostics and sex-specific therapy.
Cardiac AI: Patient-specific disease models for Precision Therapy
The goal of Cardiac Artificial Intelligence (AI) is to develop precision therapy for heart failure. Here, we use human heart slices from non-transplantable donor hearts. Various drugs affecting different ion channels are used to model patient specific affects. This data is compared with IPSC-derived slices or “NuHearts,” created from those specific patients. Collectively, the simulations and experimental analyses will be used to train AI that will be able to predict human response to pharmaceuticals.
FUNDING
NIH
Unpinning Termination Therapy for Ventricular Tachyarrhythmias
Exploration of Arrhythmogenic Triggers and Substrates in Heart Failure
Fondation Leducq
Professor Efimov has been appointed the North American Coordinator of RHYTHM, part of Fondation Leducq’s Transatlantic Networks of Excellence Program. The network, comprised of leading experts in the U.S. and Europe, will study mechanisms of sudden cardiac death to generate new clinical tools for characterizing abnormal repolarization, and to provide novel high-resolution imaging tools and risk stratification parameters for personalized preventive therapy.
COLLABORATIONS
John Rogers, in the Department of Materials Science and Engineering at Northwestern University
Natalia Trayanova, in the Department of Biomedical Engineering at the Johns Hopkins University.
Dmitry Kireev, in the Department of Biomedical Engineering at University of Massachusetts Amherst.
Luyao Lu, in the School of Engineering & Applied Science at George Washington University
Ivan Moskowitz, in the Department of Pediatrics at the University of Chicago
Gina Adam, in the School of Engineering & Applied Science at George Washington University
Shana Kelley, in the Biomedical Engineering Department at Northwestern University
Bjorn Knollmann and Vanderbilt group, in the Medicine & Pharmacology at Vanderbilt University
Chris Weber, in the Pathology department at the University of Chicago
Magdalena Harakalova, in the Utrecht Young Academy at Utrecht University
Philip Gutruf, in the Biomedical Engineering department at the University of Arizona
David Pospíšil, in the Department of Internal Medicine and Cardiology at Masaryk University
Oleg Gusev, at the Life Improvement by Future Technologies (LIFT) Center at Juntendo University.
Roman Syunyaev, at the University of Nottingham
Ruslan Deviatiiarov, at the RIKEN Institute, Juntendo University Japan
Tatiana Tatarinova, in the Computational Biology Department at University of La Verne
Niels Voigt and Richard Solano, in the Molecular Pharmacology department at University of Göttingen
Peter Yingxiao Wang, in the Department of Biomedical Engineering, University of Southern California
Hangbo Zhao, Department of Aerospace/Mechanical/Biomedical Engineering, University of Southern California
Guillermo Ameer, in the Department of Biomedical Engineering at Northwestern University
Anastasia Khvorova, in the RTI and Program in Molecular Medicine at UMass Chan Medical School
Vadim Backman, in the Department of Biomedical Engineering at Northwestern University
Elizabeth McNally, at the Bluhm Cardiovascular Institute at Northwestern University
Anna Pfenniger, in Cardiology at the Feinberg School of Medicine, Northwestern University
Megan C. King, in Cell Biology at the Yale School of Medicine
Manu Beerens, in the Institute of Clinical Chemistry and Laboratory Medicine at UKE Hamburg
Sheng Xu, in the Department of NanoEngineering at University of California San Diego
Brian Barnes & Oivind Toien, in the Institute of Arctic Biology at the University of Alaska Fairbanks.
Kedar Aras, in the Department of Physiology and Biophysics at the University of Buffalo
Luisa Iruela-Arispe, in the Department of Cell and Developmental Biology at Northwestern University.
Huiliang (Evan) Wang, in the Department of Biomedical Engineering at University of Texas Austin.
Dominic Fullenkamp, in Cardiology at the Feinberg School of Medicine
Daniil Aksenov, in the Department of Radiology at NorthShore University Health System.
Gideon Koren, at Brown Medical School
Donald Buck, Founder at Buck Surgical
Sihong Wang, in Molecular Engineering at Chicago Pritzker School of Molecular Engineering
Paul Schumaker, in Pediatrics at Lurie Children’s Hospital of Chicago
Daniel Watterson, in Molecular Biology/Biochemistry at Northwestern University Feinberg School of Medicine